Xport-A functions as a chaperone by stabilizing the first 5 transmembrane domains of Rhodopsin-1

The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends - Journal of Biological Chemistry

EMC is required for biogenesis and membrane insertion of Xport-A, an essential chaperone of rhodopsin-1 and the TRP channel
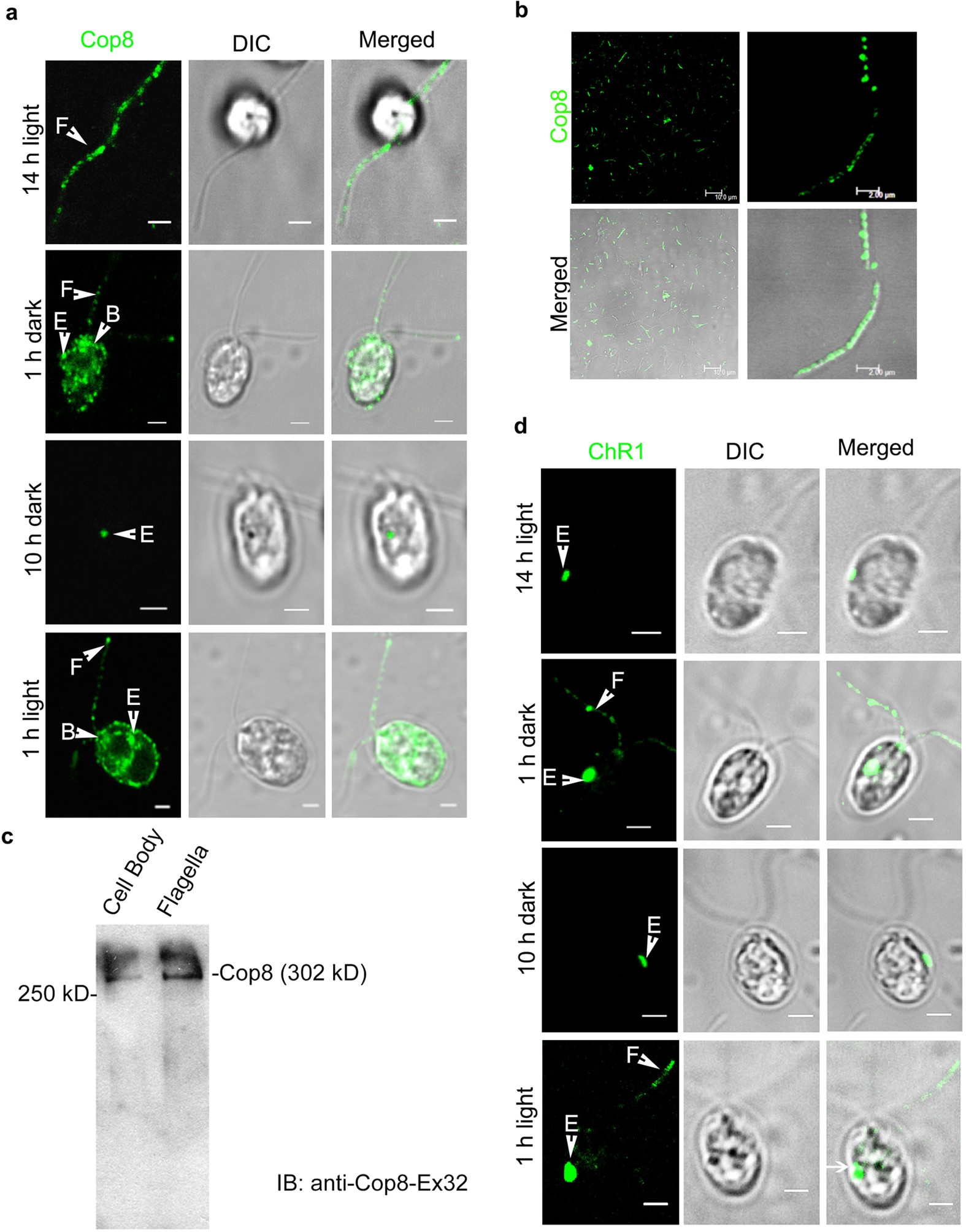
The trafficking of bacterial type rhodopsins into the Chlamydomonas eyespot and flagella is IFT mediated

ER complex proteins are required for rhodopsin biosynthesis and photoreceptor survival in Drosophila and mice

Structure network-based landscape of rhodopsin misfolding by mutations and algorithmic prediction of small chaperone action - Computational and Structural Biotechnology Journal

Rhodopsin biosynthesis defects in santa maria¹. (A) Previously proposed

Deletion of Emc1 in photoreceptor cells causes retinal degeneration in mice - Li - 2023 - The FEBS Journal - Wiley Online Library

Tiago Lopes Gomes (@TiagoLopesGomes) / X

Tiago Lopes Gomes on LinkedIn: Xport-A functions as a chaperone by stabilizing the first five…

EMC is required for the biogenesis of Xport-A in mammalian cells. A

Xport-A functions as a chaperone by stabilizing the first five transmembrane domains of rhodopsin-1 - ScienceDirect