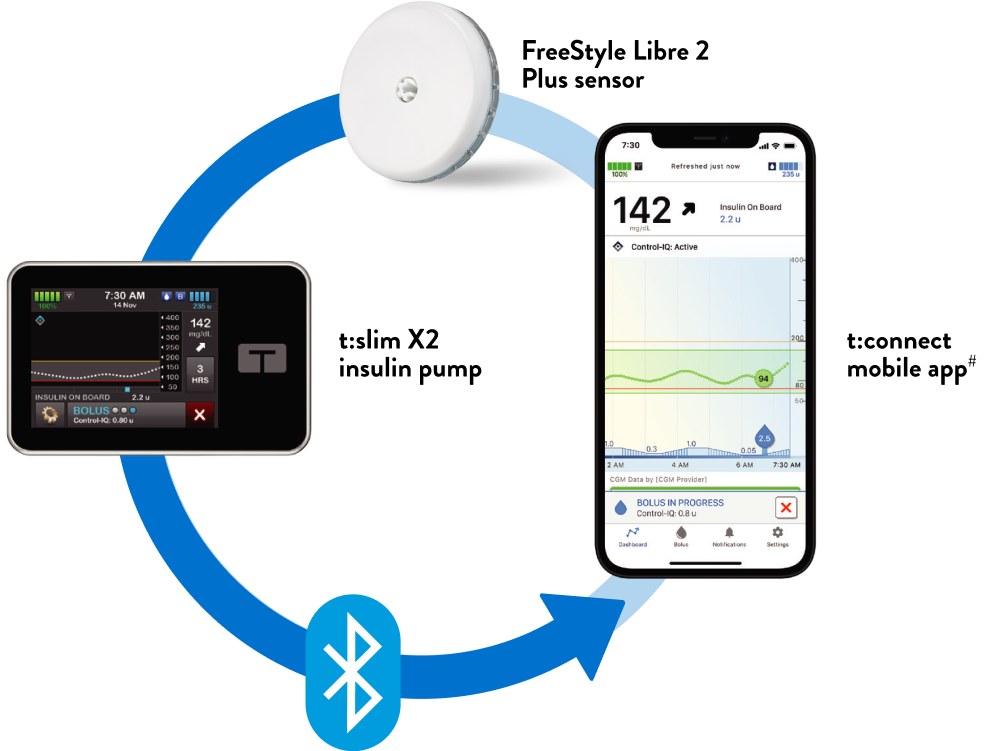
Voluntary Urgent Medical Device Correction Notice for FreeStyle Libre

Improper Charging of FreeStyle Libre Readers Prompts Alert From Abbott
FDA warns of risk of overheating, fire with some FreeStyle Libre glucose monitors
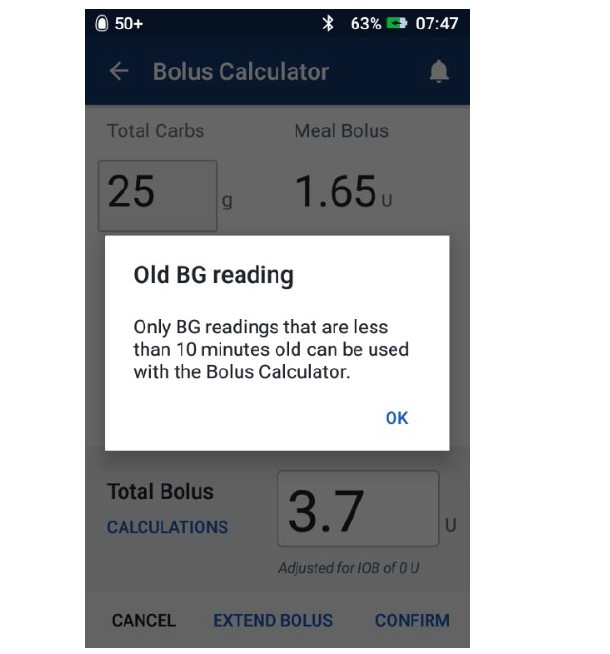
Omnipod® improper access control

Diabetes in America with Aaron Neinstein, MD, Rita Rastogi Kalyani, MD, MHS & Jennifer Raymond, MD, MCR - The Washington Post

Abbott's Freestyle Libre system becomes first CGM to be FDA cleared for use without fingersticks

424B4

Abbott's FreeStyle Libre 2 CGM picks up Medicare coverage
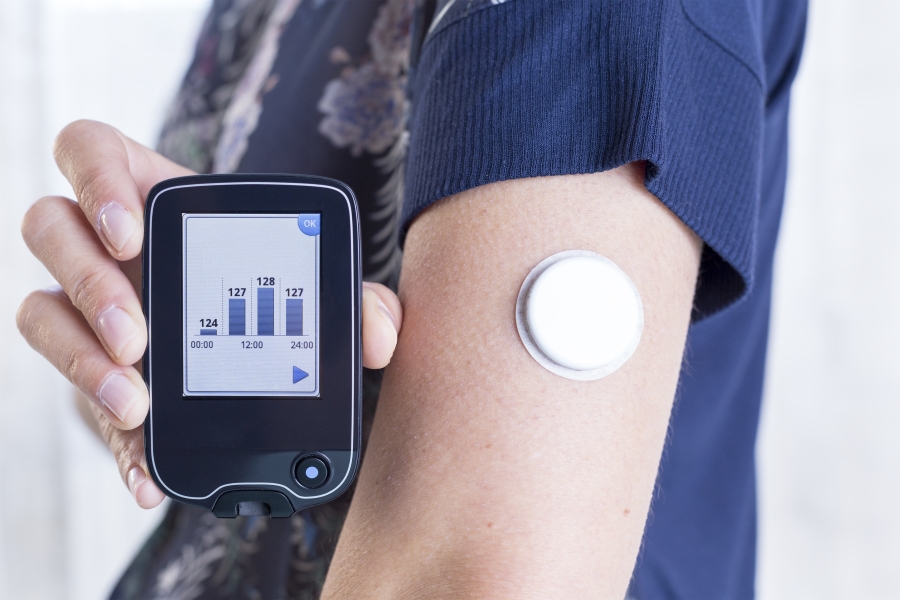
Diabetes Products Shield HealthCare

Budget Impact Analysis of the FreeStyle Libre Flash Continuous Glucose Monitoring System® in Patients with Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus with Multiple Daily Insulin Injections in Argentina

In the News.. Medtronic safety alert, Omnipod 5 in Europe, T1D Index launched and more! - Diabetes Connections

Skin Patchable Sensor Surveillance for Continuous Glucose Monitoring

2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals - Varma - 2021 - Journal of Arrhythmia - Wiley Online Library

FreeStyle Libre 2 Sensor - Healthcare Home Medical Supply USA